Many nematode species are capable of displaying a vast array of surprisingly complex behaviours. In Pristionchus pacificus one of the most striking behaviours, can be observed while feeding, as P. pacificus is a highly efficient predator, able to kill and feed upon the larvae of other nematodes to supplement its bacterial diet. As these feeding behaviours are absent from the model organism Caenorhabditis elegans, we are exploiting the P. pacificus system to investigate the evolution of novel behaviours and the nervous system itself. Predatory feeding in P. pacificus is dependent upon the phenotypically plastic but fixed mouth types termed the eurystomatous and stenostomatous morphs with only eurystomatous animals capable of predatory activities. In order to successfully engage in predatory feeding, P. pacificus also demonstrates a feeding mode switch whereby pharyngeal pumping is slowed and its teeth are stimulated, characteristics which are absent during bacterial feeding.
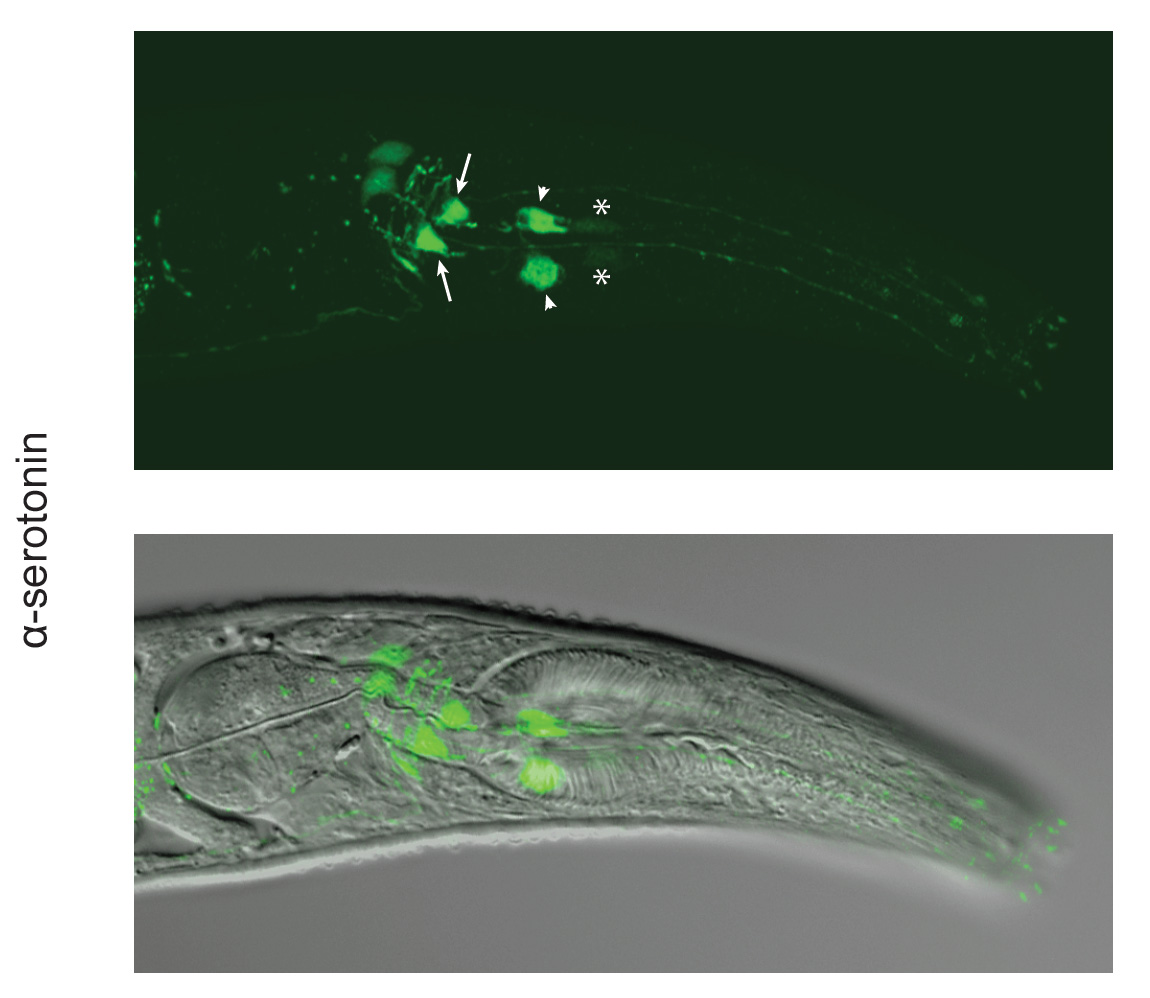
In order to unravel the mechanisms regulating predation, we are combining connectomic, pharmacological, genetic and molecular approaches. Thus far we have revealed fundamental differences in the pharyngeal wiring between P. pacificus and C. elegans with several neurons implicated as candidates for regulating these behavioural modifications (Bumbarger et al., 2013). Additionally, we have identified a key role for the neurotransmitter serotonin (Wilecki et al., 2015), which we are currently investigating further through CRISPR generated mutations in crucial enzymes involved in its synthesis and transport. Finally, recent data has revealed novel mechanisms influencing prey selection, with extreme levels of specificity evident. Therefore, through these comparative studies of the neural mechanisms regulating the feeding behaviours of C. elegans and P. pacificus, we will provide insights into the evolution of complex behaviours and the corresponding modifications of the nervous system that facilitates these actions. This work is continued in the laboratories of Dr. Misako Okumura and Dr. James Lightfoot.
Laborjournal Article: Mutterliebe geht durch die Nase (PDF)
Lightfoot, J. W., Wilecki, M., Roedelsperger, C., Moreno, E., Susoy, V., Witte, H. & Sommer, R.J. (2019): Small peptide mediated nematode self-recognition prevents cannibalism. Science, 364, 86-89.
Moreno, E., Lightfoot, L. J., Lenuzzi M., & Sommer, R. J. (2019): Cilia drive developmental plasticity and are essential for efficient prey detection in predatory nematodes, Proc. Roy. Soc. B, 286, 1343.
Okumura, M., Wilecki, M. & Sommer, R. J. (2017): Serotonin drives predatory feeding behavior via synchronous feeding rhytms in the nematode Pristionchus pacificus. G3, 7, 3745-3755.
Lightfoot, J., Wilecki, M., Okumura, M. & Sommer, R. J. (2016): Assaying predatory feeding behavior in Pristionchus and other nematodes. Jove., J. Vis. Exp., 115: e54404.
Wilecki, M., Lightfoot, J. W., Susoy, V. & Sommer, R. J. (2015): Predatory feeding behaviour in Pristionchus nematodes is dependent on phenotypic plasticity and induced by serotonin. Journal of Experimental Biology, 218, 1306–1333.
Bumbarger, D. J., Riebesell, M., Rödelsperger, C. & Sommer, R. J. (2013): System-wide rewiring underlies behavioral differences in predatory and bacterial-feeding nematodes. Cell, 152, 109-119.